Hello user, In the last article I have discussed the Mechanical Properties of Material you can read that article. But Today I will discuss other properties which are Thermal properties of Material.
Before moving to our main topic let us have the meaning of the word ‘Thermal’ first, The word ‘Thermal’ means Temperature and Heat.
What are Temperature and Heat?
The temperature definition is, Temperature is a degree of measuring the hotness and coldness.
Whereas Heat is the energy that is transferred across the boundary of the system to change the difference between the system and surroundings.
Note: Heat and Temperature are two different quantities. What we are giving in the water is called Heat and the rising in the water is called Temperature.
E.g. I have taken a metal and kept it in the sun, then I am giving in heat and the increase in the level of degree of hotness and coldness is temperature.
So these are the way that specific material will change the temperature and their states.
Now coming to our main topic Thermal Properties of Material,
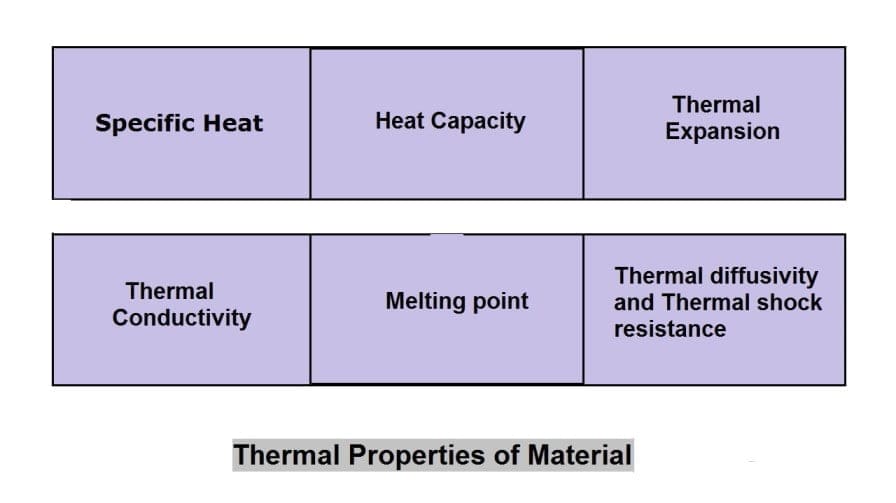
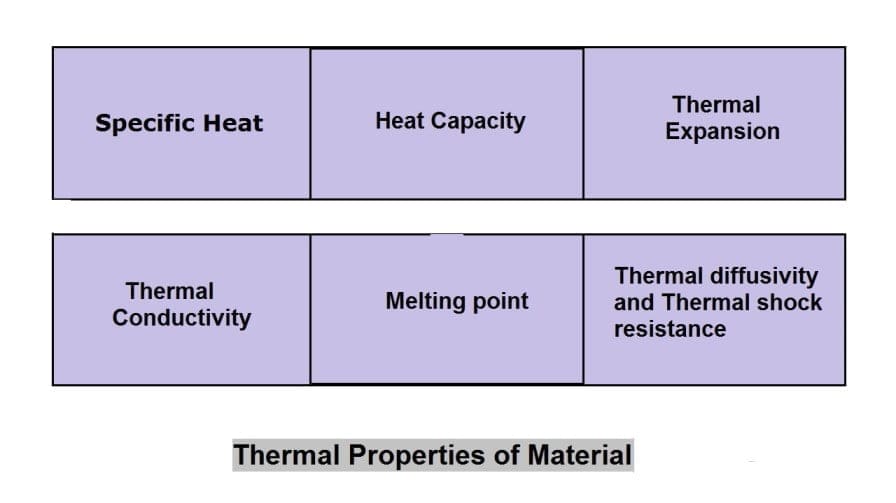
Thermal Properties of Material:
The different thermal properties of Material are following:
- Specific Heat
- Heat capacity
- Thermal Expansion
- Thermal conductivity
- Melting point
- Thermal diffusivity and
- Thermal shock resistance.
Specific Heat:
Specific heat is a property that tells which material gets heated very quickly. So Specific heat is the quantity of heat that we need to change 1 unit degree in unit mass. It is denoted by ‘S’ and the unit is joule/kg Kelvin.
Mathematical Expression,
(1/m)*(∆Q/∆T)
Where
- m= Mass
- T= Temperature
- Q = Energy content
- Note: Specific heat varies for all materials
For example, We use water instead of ice as a coolant in the radiator because the water’s specific heat is higher, the water can absorb more heat as a comparison to ice.
So Specific heat has varied by changing the phase
SP. The heat of water = 1
SP. Heat of Ice = 0.5
SP. Heat of vapor = 0.4
Heat capacity:
The Heat capacity is the property of the material by which the material is known by absorbing the heat inside itself, and it tells how much heat energy we need to bring in 1-degree unit change.
In this, we say the amount of heat needed to change the entire substance to 1 degree. It is denoted by (H) and the Unit is joule/Kelvin.
Mathematical expression :
H= m*s= ∆Q/∆t
Thermal Expansion:
Whenever we put heat energy in a material, it expands, which we call it thermal expansion. That is, there is a change in his dimension and this change comes in all directions.
If we see it in the linear direction, then we will call it the Linear Expansion, If you look in an area, then there will be area expansion, if you talk in volume, then it will say volume expansion.
Thermal expansion depends on interatomic forces. On increasing the temperature, the space between the molecules increases, which also increases their dimension.
Because we gave more temperature and its energy increased which also caused vibration in the molecule and it started expanding.
There are three types of thermal expansion:
- Linear expansion
- Superficial expansion
- Cubical expansion
Thermal conductivity:
Thermal conductivity is the property of materials that helps heat energy flow easily. This identifies the ability of the material to cause heat conduction in these materials.
How much thermal material is allowing the material to conduct heat, means how much of it is being allowed to pass the heat from the Material. It is called the thermal conductivity of the material.
It is denoted by ( K ) and the unit is watt/ meter Kelvin.
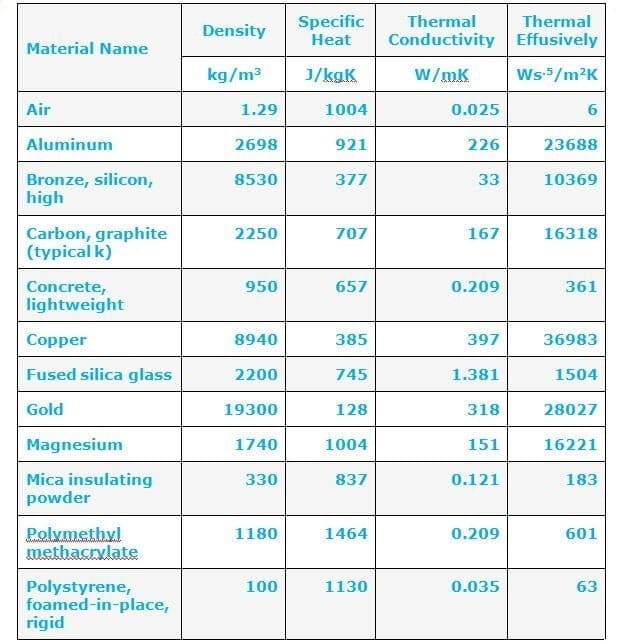
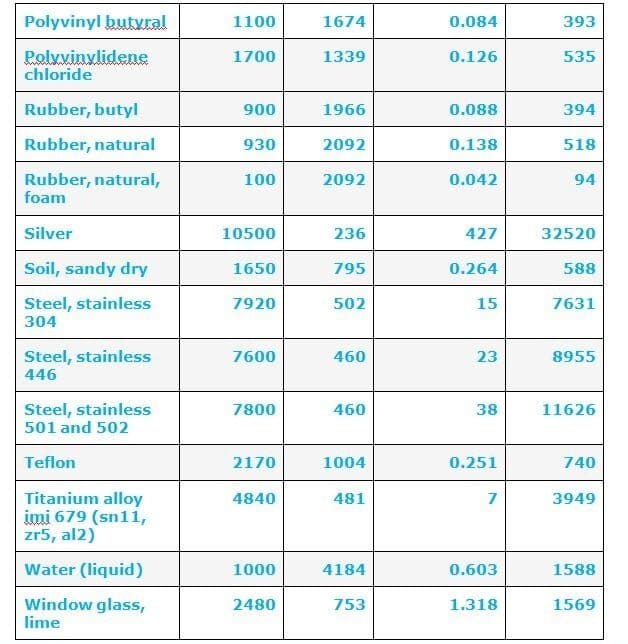
The value of thermal conductivity depends on solid liquid and gas.
Thermal conductivity of solid = 407.0 w/mk
Liquid= 0.51 w/ mk and
Gas = 0.022 w/mk
So It shows that the Thermal conductivity of solid ( Ks ) is greater than the thermal conductivity of liquid (Kl) and gas (Kg).
The metal has the highest thermal conductivity, the alloy is less than metal, and the lowest is non-metal.
Effect of thermal conductivity in solid, liquid, and gas:
In solid, Pure solid metal has the highest thermal conductivity because pure metal has free-electron present, Which works to transfer heat by gaining heat,
While fluid liquid gas has low thermal conductivity due to inter-molecular collagen.
Effect of temperature on thermal conductivity of solids:
As the temperature of the solid increases then thermal conductivity decreases in solids. As the metal is heated, the temperature will increase and it will transfer less heat from it.
But In aluminum and uranium, as the temperature increases, thermal conductivity also increases. that’s why we use aluminum in the kitchen.
Effect of temperature on thermal conductivity of fluids:
The liquid’s thermal conductivity will decrease as the temperature will rise in the liquid, because their density continuously decreases.
But water exception is the case, it does not happen with water, if the temperature increases in water, thermal conductivity will also increase.
Effect of temperature on thermal conductivity of gases:
The thermal conductivity of the gases will increase as we increment the temperature. As the molecular weight of the gases increases, the thermal conductivity decreases.
Melting point:
The melting point is the temperature of a material where it changes from a solid phase to a liquid phase. The melting point of pure metal is fixed while it varies for alloys.
It also depends on its bonding forces How strong is the bonding force, the higher the melting point. Covalent bonds have the highest melting point than ionic, and last metallic molecular bonds.
A low melting point is used for safety devices like fuse wire, fuse plug, and boiler safety devices.
Thermal diffusivity:
The ratio of our thermal conductivity and heat capacity is called thermal diffusivity. In thermal diffusivity, it tells us, if we supply heat to the material, then how soon it will distribute the heat to the other end of the material.
This means if the diffusivity of our material is very high, then the supply of heat to the material will distribute the heat at high speed.
E.g. We have a rod, we heat it from a corner, so its temperature is 100 degrees there.
So if our thermal diffusivity is high, our metal will be 100 degrees Celsius at the other end also in a short time. Means will distribute the heat very quickly.
Material having a high value of thermal diffusivity acknowledges very quickly to change in the thermal environment for set up the steady-state.
Note: Heat capacity, Thermal inertia, and Thermal capacitance all are the same terms.
Thermal shock resistance:
This is the condition when sudden Change occurs in the temperature of the material.
If a body remains the same without failure after sudden temperature, then we call that ability thermal shock resistance.
E.g. Thermal shock resistance is higher in the ductile material as compared to a brittle material.
Related Article:
- Venturimeter
- Orifice meter
- Valve Types
- Forging vs Casting
- Hot Working Process
- Cold Working Process
- Forging Tools and their Uses
- Centrifugal Casting
- Buffing and Polishing Process
- Grinding Machine
- Lathe Machine
- Milling Machine
- Drilling Machine
- Planer Machine
- Slotter Machine
So here we finally studied all the material properties of thermal. If you have any doubt let me know in the comment section. And if you found it helpful then please share it on social places like Facebook, WhatsApp.



Discussion about this post