In the year 1911 Electrochemical machining technique was first discovered by a Russian chemist E. Shpitalsky Which began to get better day by day. The history of electrochemical grinding is unknown because electrochemical grinding is a variation of electrochemical machining.
Hence we can say that electrochemical grinding was discovered experimentally while working with ECM. However, some say that electrochemical grinding became popular in the 1930s in the USA and was then used for grinding carbide in the 1950s.
Nowadays electrochemical grinding is a common process that has many advantages over traditional grinding methods.
In this article, we will study the Definition, Parts or Construction, Working principles, Application, Advantages, and Disadvantages of Electrochemical Grinding in detail.
Note: At the end you can download PDF file easily.
Let’s start with the definition first,
What is the Definition of Electrochemical Grinding?
The term grinding refers to a machining process in which the material is removed from the surface of the workpiece. And the term electrochemical resembles the mode of energy used for the machining process. Electrochemical grinding is a combination of electrochemical machining and grinding processes.
The major difference between ECM and ECG is that In ECG grinding wheel is used instead of a cutting tool. Electrochemical grinding is done when there is a need to remove the material from the surface of the workpiece. It is generally used when the mechanical or traditional grinding of a workpiece takes a lot of effort due to the hardness of the workpiece.
Hence it can be said that electrochemical machining is used for grinding materials with greater hardness (greater than 65 HRC).
Electrochemical grinding is a reverse of electroplating. In this, the metal surface is converted into its respective oxide which is then removed by the rotating grinding wheel.
Only 5-10% of the material is removed by the Abrasive grinding wheel, the rest is removed by the flow of electrolyte.
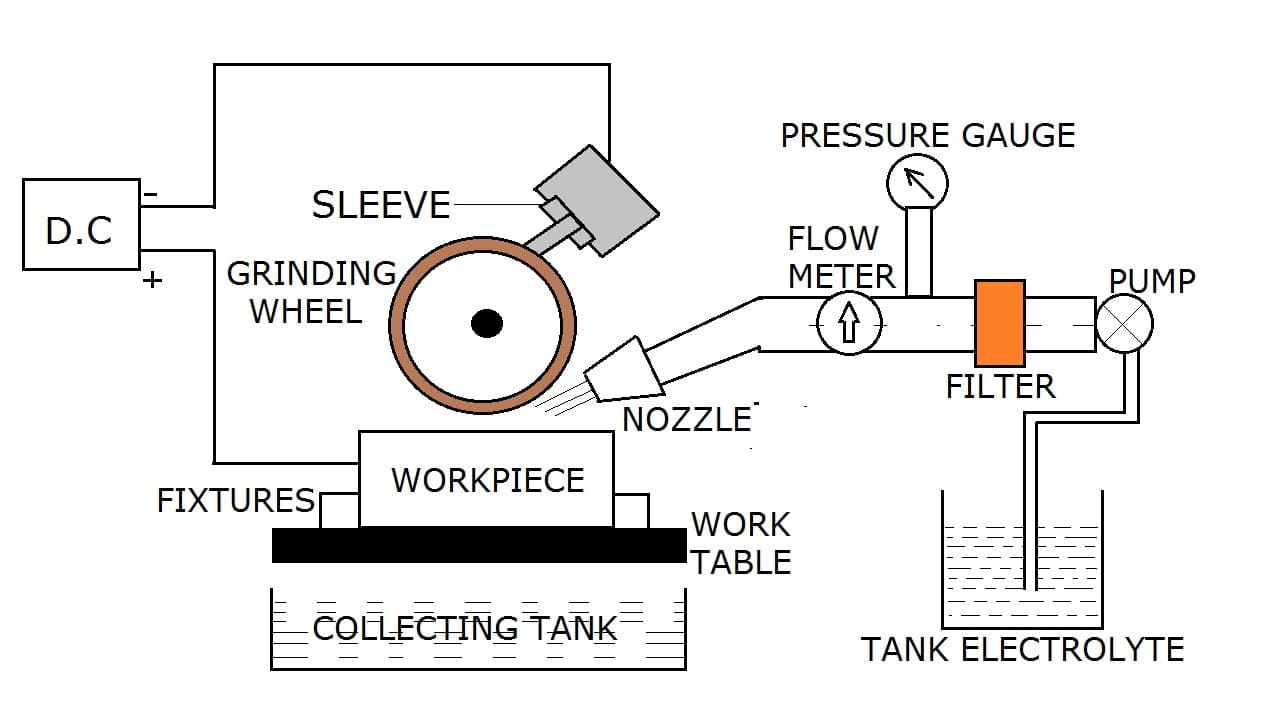
Parts or Construction of Electrochemical Grinding:
The workpiece is kept on the worktable which acts as a rigid base. There are fixtures located on the table to clamp the workpiece. Below the worktable, a collecting tank is situated for the collection of waste electrolytes. The workpiece is connected to the positive terminal of the power supply and acts as an anode while the grinding wheel acts as the cathode. The power supply to the grinding wheel is given through the sleeve. The supply line consists of an electrolyte tank. The pump is used to draw the electrolyte from the tank which is followed by the filter, pressure gauge, and flow meter. The end of the supply line is connected with a nozzle for increasing the pressure.
Various parts involved in an electrochemical grinding setup are as follows:
- DC power supply
- Work Table and fixture
- Electrolyte tank
- Pump
- Filter
- Pressure Gauge and flow meter:
- Nozzle
- Sleeve
- Grinding wheel and
- Collecting tank
Now we will study one by one in detail.
DC Power supply:
A DC power supply with low voltage and high current is used for providing electrical energy to the setup. The voltage is kept low to avoid the generation of heat and for safety purposes as well. High current on the other hand promotes a faster process.
Work Table and Fixture:
For any machining process, it is important to provide a rigid base and restrict all the degrees of freedom of the workpiece. The worktable provides rigid support to the workpiece and fixtures are used to clamp the workpiece.
Electrolyte tank:
An electrolyte tank is a reservoir in which the electrolyte is stored. An electrolyte is a conducting solution that plays an important role in electrochemical grinding. The first function of electrolyte is to complete the circuit by providing a conducting medium.
The second function is to oxidize the metal surface and carry out the oxidized particles. Electrolytes are generally Sodium compounds formed by electrovalent bonds. There are various types of electrolytes used in electrochemical grinding such as Sodium nitrate (generally used), carbonate, hydroxide, and Sodium chloride.
Pump:
A pump is used to carry the electrolyte from the electrolyte tank to the nozzle. The pump used is generally electrically driven.
Filter:
The electrolyte passes through a filter where all the micro impurities get filtered and pure Electrolyte is obtained.
Pressure Gauge and flow meter:
Pressure gauge and flow meter are safety equipment that shows pressure and flow of the electrolyte respectively. If any of these values become more than the safety limit the operator simply turns off the equipment.
Nozzle:
A nozzle is a device with decreasing cross-section area, which is used to direct the electrolyte to the correct position. The decreasing area helps in increasing the velocity of the electrolyte which in turn removes the material from the workpiece.
The nozzle is placed in such a way that the electrolyte flowing out of it must come in contact with the workpiece as well as the grinding wheel.
Sleeve:
A sleeve is used to transfer electrical energy to the grinding wheel.
Grinding wheel:
The grinding wheel is the most important and unique part of the electrochemical grinding machine. It is connected to the negative terminal of the power supply and acts as the cathode.
The grinding wheel is made of insulating materials such as diamond and aluminum oxide. The wheels rotate and increase the flow of the electrolyte.
The grinding wheel is responsible for only 5-10 % of the material the rest is removed by the electrolyte. There is hardly any contact of the grinding wheel with the workpiece hence no or very little wear takes place.
Collecting tank:
A collecting tank is used to collect all the used electrolyte which is then disposed or reused as per the requirement.
Electrochemical grinding Working In detail:
Before understanding the working we must go through the principle of working of electrochemical grinding.
The working principle of electrochemical grinding is When a metal surface is acted upon with an electrolyte under a high current, the metal surface gets oxidized to form an oxide layer (corrosive layer). This layer is removed with the action of a flowing electrolyte and rotating grinding wheel. Metal is removed in the form of an oxide layer to obtain an n surface finish.
Now we will study step by step in detail,
The workpiece to be machined is first kept on the worktable and then clamped using fixtures.
The grinding wheel is placed at the required position. A gap is maintained between the workpiece and the grinding wheel. This gap is generally 0.02mm.
Now the power supply is switched on. The pump is then driven to supply the electrolyte to the required position.
At first, the electrolyte passes through a filter where all the impurities are filtered. Then the electrolyte is passed through a pressure gauge where the operator checks for the correct pressure.
Then electrolyte is passed through a flow meter where flow is checked by the operator.
After passing through all the stages the electrolyte reaches the nozzle. Nozzle increases the velocity of the electrolyte and sprays it over the workpiece.
As soon as the electrolyte comes in contact with the workpiece and the grinding wheel, the circuit is completed which results in Oxidation of the metal surface.
This forms a layer of oxide which is removed by the flow of electrolyte and the Abrasive particles in the grinding wheel. After the grinding is done, the flow is stopped and the power supply is switched off.
The workpiece is then unclamped and the remaining electrolyte is wiped out from the surface.
Electrochemical Grinding Working Video:
Applications of Electrochemical Grinding:
The following uses or applications of Electrochemical Grinding are:
- ECG is used for grinding turbine blades.
- It is used in aerospace industries for grinding honeycomb.
- Also used for finishing hard surfaces.
- It is also used for creating sharp objects.
- It is also used for grinding fragile articles.
Advantages of Electrochemical Grinding:
The following merits or advantages of Electrochemical Grinding are:
- The accuracy of electrochemical grinding is very high. As there is no contact of the tool with the workpiece.
- A high tolerance can be obtained.
- The process does not leave any scratches on the surface of the workpiece.
- The heat generated during the operation is very less.
- The electrolyte also acts as a coolant.
- There are no internal stresses developed in the workpiece.
- Hard materials can be grinded easily.
- Cleans edges can be obtained.
- No or very little wear of the grinding wheel takes place.
Disadvantages of Electrochemical Grinding:
The following demerits or disadvantages of Electrochemical Grinding are:
- The metal removal rate is low, about 15mm/s
- Power consumption is very high as there is a need to drive the grinding wheel and pump as well.
- The initial cost of the equipment is also high.
- The production rate is low.
- Disposing of waste electrolytes becomes a problem, which makes the process non-eco-friendly.
- A large area is required for setup.
Related Resources:
- Abrasive Jet Machining
- Ultrasonic Machining
- Laser Beam Machining
- Electrochemical Machining
- Grinding Machine
- Lathe Machine
- Milling Machine
Reference:
- Open.edu
- Youtube Video: LEARN AND GROW
Conclusion:
So here we have studied Electrochemical Grinding in detail. Let me know what else I can help you in this topic or any other topic?. You can check our another article I am sure that can boost your knowledge. Till then Thank you for reading.






Discussion about this post