What is Steel?
Steel is an alloy typically consisting of iron and carbon along with small amounts of manganese, silicon, phosphorus, sulfur, and oxygen. The amount of carbon content present ranges from 0.02% to 2.14% and the presence of manganese is around 1%.
Other than these, the common alloying elements include manganese, cobalt, boron, nickel, chromium, molybdenum, titanium, vanadium, tungsten, and niobium.
The density of steel is in the range between 7,700 and 8,050 kg/m3. The presence of carbon creates a strong molecular structure. It can take two crystalline forms, body-centered cubic, and face-centered cubic depending on the temperature.
Pure iron is often ductile, soft, or easily formed due to the iron atoms in the crystal structure slipping past one another.
Here the presence of carbon and other elements act as hardening agents which prevent the dislocation of atoms. As a result, the amount of carbon and other elements present in the alloy makes up for the final physical qualities of the alloy.
These qualities include quenching behavior, need for annealing, tempering behavior, hardness, tensile strength, and yield strength. Evident production of steel dates back to the sixth century BC, produced in South India known as Wootz steel.
Steel Overview
Modern evolution led to the use of blast furnaces which was followed by the use of electric furnaces for processing steel. Steel making process consists of a primary process that involves the production of pig iron. It is melted iron, from iron ore which contains more carbon than optimum for steel.
An oxidization process is used to remove excess carbon and also to vaporize or bind impurities made of elements like silicon, phosphorus, and manganese.
The secondary steel-making process involves refining and alloying the steel. It can either initialize with the use of scrap steel or be a continuation of the primary process.
This is among the world’s most important engineering and construction materials as it is used in every aspect of our lives.
Cars and construction products, refrigerators and washing machines, cargo ships, and surgical scalpels require steel for manufacturing. It can be recycled over and over again without loss of properties.
World crude steel production is estimated to reach 1,990 million tonnes (Mt) in the year 2022. Ideally, steel is magnetic except for stainless steel (chromium alloyed steel).
Ferromagnetism is dependent on the alloying element in this case. Completely recyclable and durable compared to other materials, it requires relatively low amounts of energy to produce.
It is among the most recycled materials in the world with a recycling rate of over 60%. China, Japan, Russia, and the US respectively are the largest producers of steel worldwide.
Properties of Steel
#1. Tensile Strength
The tensile strength of a material is defined as the amount of stress that the element can undergo before deforming structurally. The tensile strength of steel is comparatively high, thus making it highly resistant to fracture or breakage.
#2. Ductility
Ductility is the mechanical property, which describes the ability of an element to change its shape upon application of force to it without resulting in fracture. Steel is highly ductile and is used in producing different shapes and structures ranging from thin wires or large automotive parts and panels.
#3. Durability
Since steel has high hardenability due to the presence of carbon, it reflects its ability to resist strain. It is highly resistant to external wear and tear which makes steel a highly durable material.
#4. Malleability
Malleability is the ability of an element to be compressed into thin sheets and allows the steel to be deformed under compression. Steel can be converted into sheets of variable thicknesses, often created by hammering or rolling.
#5. Conductivity
Steel is an excellent conductor of heat and electricity. As a result, it is a preferred choice in the household cookware industry along with the electrical wiring industry.
What are the Different Types or Classification of Steel?
Various different types of Steel are manufactured with variable carbon content from 0.2 to 2.1 percent by weight, classification depending upon the composition and their physical properties.
Even though the major element in steel is carbon, other alloying elements i.e. tungsten, chromium, vanadium, and magnesium, and a small amount of sulfur, silicon, phosphorus, and oxygen are also present.
The amount of alloying materials and their form of presence in iron decides important properties of steel like ductility, hardness, and tensile strength.
For example, increasing the amount of carbon makes the steel hardened and strong, but less ductile.
Classification of Steel is much more complicated due to its many properties and applications. Then too, comprehensive grading systems have been developed to accurately identify a particular type of steel within groups and subgroups.
The major types of steel are explained in detail below:
#1. Carbon steel
Carbon steel generally consists of less than 2 % carbon along with traces of manganese, sulfur, silicon, and phosphorus.
The characteristics of carbon steel are mainly influenced by the carbon content in steel and alloying elements cause only negligible changes.
Plain carbon steel can be further classified into four categories depending on the amount of carbon present.
The detailed classification is as follows:
Low Carbon Steel
In low-carbon steel types of steel, the amount of carbon is limited to 0.30. It is the most commonly used grade.
This type can be machined and welded easily. It also has ductility higher than high-carbon steel. These are used in pipes, bolts, and wires.
Medium Carbon Steel
In medium carbon steel, the amount of carbon content present is between 0.30 to 0.45 %. The increase in the amount of carbon content indicates an increase in hardness and tensile strength and a decrease in ductility.
But, because of the higher carbon content, its machining and welding properties become lower than low-carbon steel due to the increased hardness. These are found in gears and railroad tracks.
High Carbon Steel
High carbon steel contains 0.45 to 0.75%. These types of steel have complicated welding and machining properties.
So, for any type of molding work heat treatment is necessary to produce acceptable welds. It is also used to control the mechanical properties of steel after welding.
Very High Carbon Steel
In very high-carbon steel, the amount of carbon content present is above 0.61% and goes up to 1.50%. To deal with the high carbon content present in the steel, it requires heat treatment before, during, and after welding to manipulate its mechanical properties.
This high carbon type is used to manufacture hard steel products such as truck springs and metal cutting tools. These do not contain more than 2% carbon.
Designation system of Carbon steel
The American Iron and Steel Institute (AISI) together with the Society of Automotive Engineers (SAE) introduced a four-digit designation system for better identification of each element.
SAE 1XXX
First digit
The 1st letter of the digit indicates either it is alloy steel or carbon steel.
Number 1 indicates carbon steel and numbers 2-9 are used for alloy steels.
Second digit
The second digit is used to indicate whether the steel has been modified.
0 – Non modified plain carbon
- 1 – Resulfurized
- 2 – Resulfurized and phosphorized
- 5 – Non desulfurized, Manganese over 1.0%
Last two digits
The last two digits show carbon concentration in terms of 0.01%.
For example, SAE 1045: In which 1 indicated plain carbon (non-modified) steel and consists of 0.45% carbon.
#2. Alloy Steel
Alloy steel is a type of carbon steel, which consists of one or more elements other than carbon, added to produce the desired characteristic.
Each added element contributes its separate attribute to the final product. Generally, elements such as silicon, boron, chromium-molybdenum, manganese, nickel, and vanadium are added as external elements.
There are two types of alloy steel, namely low alloy steel and high alloy steel. The detailed explanation is as follows,
Low alloy steel
In low alloy steel, the amount of carbon content present is generally between 0.15% to 0.25 %, suitable for welding purposes.
Some of the alloying elements used are manganese, nickel, chromium, molybdenum, silicon, vanadium, and boron and the less common alloying elements are aluminum, cobalt, copper, titanium, tungsten, tin, and zirconium.
Low alloy steel is most preferably used to achieve better hardenability and increased corrosion resistance in certain environments. Difficulty to weld is a certain drawback for these types of steel.
If the carbon content is decreased up to 0.10 percent, along with other alloying materials, the strength of the material can be increased. The versatility of these makes them hem essential in modern industrial development.
Common applications include electrical wiring, heat exchanger, anti-drill plates, high-strength safes, pipelines, electrical transformers, and permanent magnets.
High alloy steel
Generally, steel having other elements more than 8% of total weight other than carbon and iron is known as high alloy steel. High alloy steel essentially has two chemical elements, one is carbon and the other is the added element.
The properties of these types of steel depend on the percentage of the chemical elements present in it. One of its major advantages is that it offers high corrosion resistance along with high reliability.
These types of high-carbon steel are extensively used in driers, pipelines, couplings, valves, bolts, salt manufacturing, nuclear power plants, heat exchangers, centrifugal separators, exhaust gas desulfurized, petrochemical, pharmaceutical, and semiconductor cleaning equipment.
Designation system for Alloy Steel
The American Iron and Steel Institute (AISI) together with the Society of Automotive Engineers (SAE) developed a four-digit designation system for better identification of alloy steels. As per the four-digit classification of the SAE-AISI system:
First digit
- The first digit indicates the class of alloy steel:
- 2- Nickel steel
- 3- Nickel-chromium steel
- 4- Molybdenum steel
- 5- Chromium steels
- 6- Chromium-vanadium steels
- 7- Tungsten-chromium steels
- 9- Silicon-manganese steels
Second digit
The second digit shows the concentration of the major element present, in percentages. If the 2nd element is 1 or 2 it means 1% or 2%, respectively.
Last two digits
The last two digits indicate carbon concentration, in terms of 0.01%.
For example, SAE 6230: is indicates an alloy of Chromium-vanadium steel, consisting of 2% chromium and 0.30% carbon.
#3. Stainless Steel
Stainless steel is a type of steel that is corrosion and rust-resistant. Invented in England in 1913 by Harry Brearley, it was announced to the world in 1915.
Chromium is the element that sets it apart, which is responsible for the materials’ luster. But it is more than just a cosmetic addiction, as chromium is oxidation resistant and thus the antirust has a direct relation with materials’ increased longevity.
Stainless steel has a chromium content of more than 10.5% and can go up to 30% in some applications. Chromium content is directly professional to the gloss when polished and the corrosion resistance. It has almost 150 types of grades, but only 15 are used commonly.
It is commonly known for its role in medical equipment and appliance manufacturing, also with kitchen appliances. Stainless steel can act differently when chromium is electroplated onto another metal and produce a tough, polished coating.
This is classified into four categories, with each serving a different purpose.
Martensitic Alloys
Martensitic alloys are extremely tough but prove to be corrosive. These are formed by a rapid cooling process, ideal for heat treatment, and are found in cutlery, medical instruments, and pliers.
Ferritic Alloys
Ferritic alloys contain low amounts of carbon and nickel. These are less expensive and are extensively used in the automotive industry because of their chromium-induced strength and sheen.
Austenitic Alloys
These consist of higher chromium and nickel content, which helps to improve the corrosion resistance and become nonmagnetic. Most commonly used in the commercial kitchen industry because of its durability and ability to be cleaned easily.
Duplex Alloys
Duplex alloys are a mixture of austenitic and terrific alloys which results in the doubling of strength while also inheriting the properties of both. Due to the presence of high chromium content, these are highly resistant to corrosion and are known for their ductility.
Designation system for Stainless Steel
The American Iron and Steel Institute (AISI) created a three-digit system for stainless steel:
- 2XXseries: Chromium-nickel-manganese austenitic stainless steels.
- 3XX series: Chromium-nickel austenitic stainless
- 4XX series: Chromium martensitic stainless steels and terrific stainless and
- 5XX series: Low chromium martensitic stainless steels.
#4. Tool and Die Steel
Tool and die steel ideally has a carbon content range between 0.7% to 1.5%. These are manufactured in carefully controlled conditions to produce the desired quality of steel.
Tool steels are heat treatable and are very high carbon steels (either carbon or alloy) possessing high hardness, strength, and wear resistance. Elements forming hard and stable carbides are added to the composition to increase the hardness of the steel tool.
Processes such as tempering, adding high heat, cooling quickly and heating make the tool steel extremely hard and heat-resistant. These are extensively used in high-impact environments and are very abrasive.
These are mainly used for making hummers, drills, cutters, shear blades, chisels, forging dies, drills, and razors.
Tool and die steels can be classified depending on their use, composition, mechanical properties, and method of heat treatment.
A variety of grades of tool and die steel are available for different application purposes. The added element plays a role in determining the particular application it’s suited for.
Air Hardening
This steel can be exposed to high temperatures without distorting due to the presence of high carbon content in this.
Water Hardening
This tool is water quenched during use and is the most affordable tooling type. Extensively used to make common tools.
Oil Hardening
This type of tool is oil-quenched during use and is exceptionally wear-resistant from slipping. It is commonly used to produce knives and shears.
High-Speed Steel
As the name suggests, these are made for extensive high-impact use and are extremely abrasive. It’s generally used in power saws and drill bits.
Hot Working
The ability to withstand extreme heat is a unique feature of this steel. It is used in forging and casting.
Shock Resistant
Carbon, silicon, and molybdenum are added in small amounts to harden this steel and make it suitable for punches and riveting tools.
Designation system for Tool and Die steel
The American Iron and Steel Institute (AISI) along with the Society of Automotive Engineers (SAE), developed a one-letter system for the identification is tool steels.
- W- water-hardened plain carbon tool
- O- oil hardening cold work alloy
- A- air hardening cold work alloy
- D- diffused hardening cold work alloy
- S- shock resistance low carbon tool
- T- high-speed tungsten tool
- M-high-speed molybdenum tool
- H- hot work tool and
- P- plastic mold tool steel
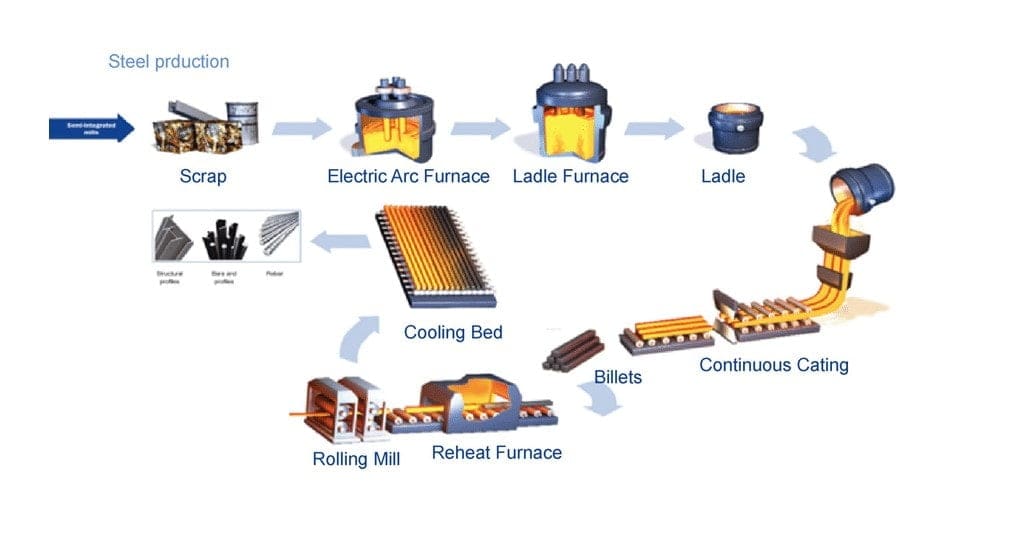
How Steels are manufactured or the Production of Steel?
Steel is manufactured via two major routes, the Blast Furnace-Basic Oxygen Furnace (BF-BOF) route and the Electric Arc Furnace route (EAF).
These are often referred to as the ‘primary’ and ‘secondary’ paths. The former method creates new or ‘virgin’ steel and the latter is often used to recycle the steel scrap.
A detailed overview of the methods is described below:
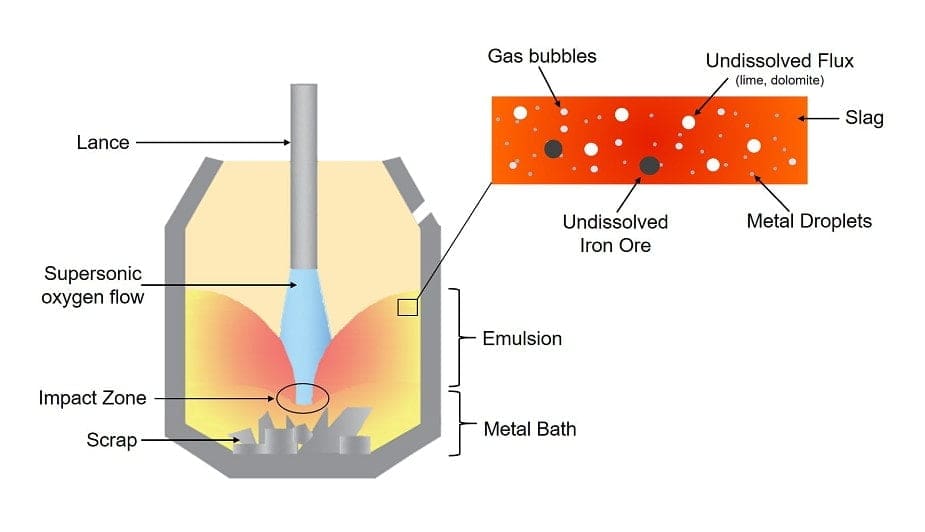
#1. Basic Oxygen Furnace
Blast Furnace Basic Oxygen Furnace Steelmaking (BF-BOF/BF-BOS) or the Linz-Donawitz-Verfahren steelmaking is an oxygen converter process. It involves both molten pig iron and steel scrap converted into steel with the oxidizing action of oxygen blown into the melt under a basic slag.
Also called the Basic Oxygen process and Basic Oxygen Converter, it is the most efficient yet powerful and effective teel-making method and it is evident in the percentage of adaptation by the industry.
It involves a reduction process, which is used to reduce the iron oxide present. A reducing agent called oxygen carbon is used to separate the iron atoms.
The oxides form a bond with the reducing agent and emit CO2. The steps of the process involved Furnace structure, Refractory lining, and Chemical, and physical processes.
Operation
BOFs consist of conventional top-blown furnaces, bottom-blown furnaces, various fixed-blowing configurations along with inert gas bottom-stirring modifications.
The top-blown basic oxygen furnace involves water-cooled oxygen for blowing oxygen into the melt through 4-6 nozzles. The flow of oxygen commonly reaches around 200-280 ft3/(min*t) (6-8 m3/(min*t)). The oxygen pressure is in the range of 150-220 psi.
The expected service life of an oxygen lance is about 400 heats. The bottom-blown basic oxygen furnace involves 15-20 tuyeres, for injecting oxygen or lime powder containing oxygen.
The tuyeres are then cooled either by hydrocarbon gas or oil supplied to the outer jacket of the tube. The global crude steel total output through the Basic Oxygen Furnace Steelmaking process is about 67% and is recognized as the dominant steelmaking technology.
Large European blast furnaces produce about 4 million tonnes per year.
Electric Arc Furnace
Electric Arc Furnace (EAF) is a steel-making process that involves a furnace in which steel scrap is heated and melted with the help of the heat of electric arcs striking between the furnace electrodes and the metal bath.
Two types of electric current may be used in Electric Arc Furnaces: direct (DC) and alternating (AC). Three-phase AC Electric Arc Furnaces containing graphite electrodes are extensively used in steel making.
A major advantage of electric Arc Furnaces over basic Oxygen Furnaces (BOF) is their capability to treat charges containing up to 100% of scrap. About 33% of steel production in the world is made in electric Arc Furnaces (EAF).
The capacity of the Electric Arc Furnace can reach up to 400 t. The core of the process are:
Structure of an Electric Arc Furnace, Refractory lining of an Electric Arc Furnace, Chemical and physical processes.
Operation of an Electric Arc Furnace
The structure of the furnace contains a spherical hearth (bottom), a cylindrical shell, and a swinging water-cooled dome-shaped roof.
The roof has three holes for the graphite electrodes held by a clamping mechanism. This mechanism provides an independent lifting-lowering of each electrode.
The water-cooled electrode holders act as a contact for transmitting electric current supplied by water-cooled cables (tubes). The three-phase current is formed by the electrode and the scrap in which the scrap is a common junction.
A tilting mechanism for tapping the molten steel through a tap hole with a pour spout located on the back side of the shell is mounted by the furnace.
The charging door is a passage through which the slag components and alloying additives are charged and it is located on the front side of the furnace shell.
The charging door is also used for de-slagging (removing slag). The furnace top commonly charges the scrap. The scrap arranged is then transferred to the furnace by a crane and then dropped into the shell.
Application or Uses of Steel:
#1. Building and infrastructure (51%)
Around half of the steel produced annually is utilized to construct buildings and infrastructure such as bridges. It is mostly found in reinforcing bars, and sheet products used in roofs, internal walls, ceilings, and structural sections.
It is also found in HVAC systems and items such as stairs, rails, and shelving.
Moreover, applications of steel in transportation-related infrastructure include tunnels, rail tracks fueling stations, train stations, ports, and airports.
#2. Mechanical equipment(15%)
The application in mechanical equipment involves machinery that makes car parts, cranes, and hand tools such as hammers and shovels along with tractors and bulldozers.
#3. Automotive (12%)
Around 2,000 pounds, or 900 kilograms, of steel, is used to make a car. About a third of that is utilized in the body structure and exterior and 23% is in the drive train, also with 12% in the suspension. A modern-day car structure contains around 60% steel of total body weight, even though the steel used is advanced high strength which is stronger and lighter.
#4. Domestic and electrical appliances (6%)
Varying amounts of steel are present in washers and dryers, microwave ovens, dishwashers, and refrigerators.
Moreover, applications in the production and distribution of electricity include transformers, which have a magnetic steel core, generators, electric motors, pylons, and steel-reinforced cables.





Discussion about this post